A focus on Prostate cancer, the second most frequent malignancy and a leading cause of cancer-related deaths in men.
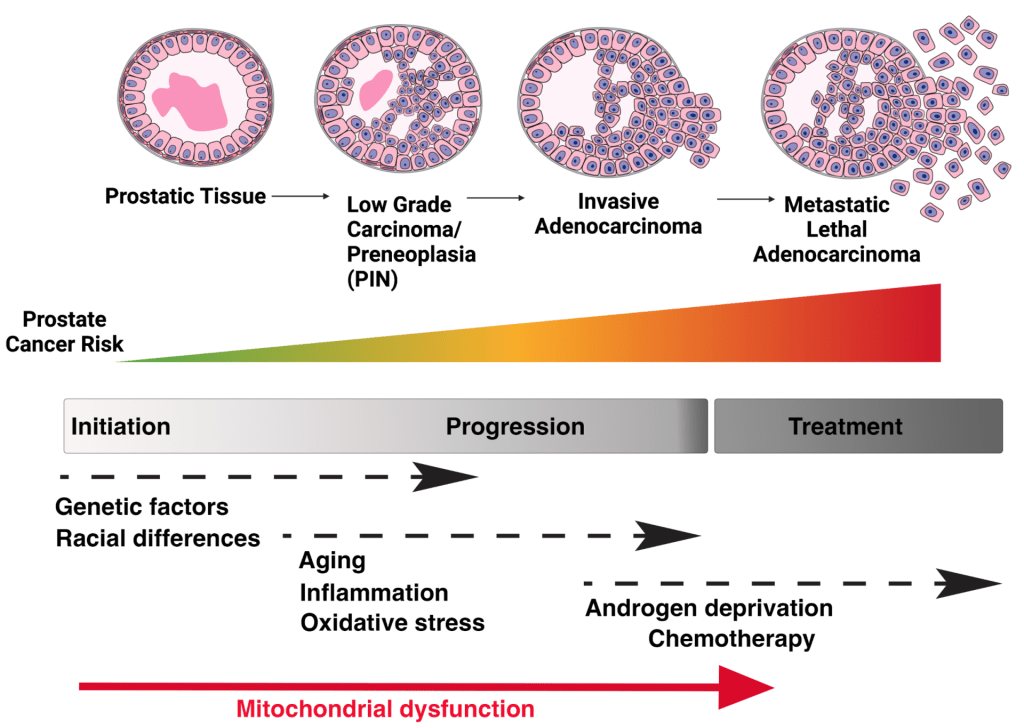
Although relatively indolent in the beginning, prostate cancer can progress to invasive and lethal disease; more often observed in African American men who we consider to be a high-risk population for aggressive disease. Several studies suggest a multifactorial etiology for such disparities, encompassing an accumulation of genetic aberrations. However, genome-based prostate cancer biomarker discovery efforts have largely focused on the nuclear genome, overlooking the smaller but essential mitochondrial genome (mtDNA). Indeed, alterations in mtDNA-encoded oxidative phosphorylation genes have been associated with increased prostate cancer risk but their exact functional impact remains unknown.
Therefore, understanding the underlaying mitochondrial determinants of prostate cancer disparities could ultimately lead to better precision interceptions and biomarkers for stratifying patients that will develop aggressive prostate cancer.
Overview and Approach
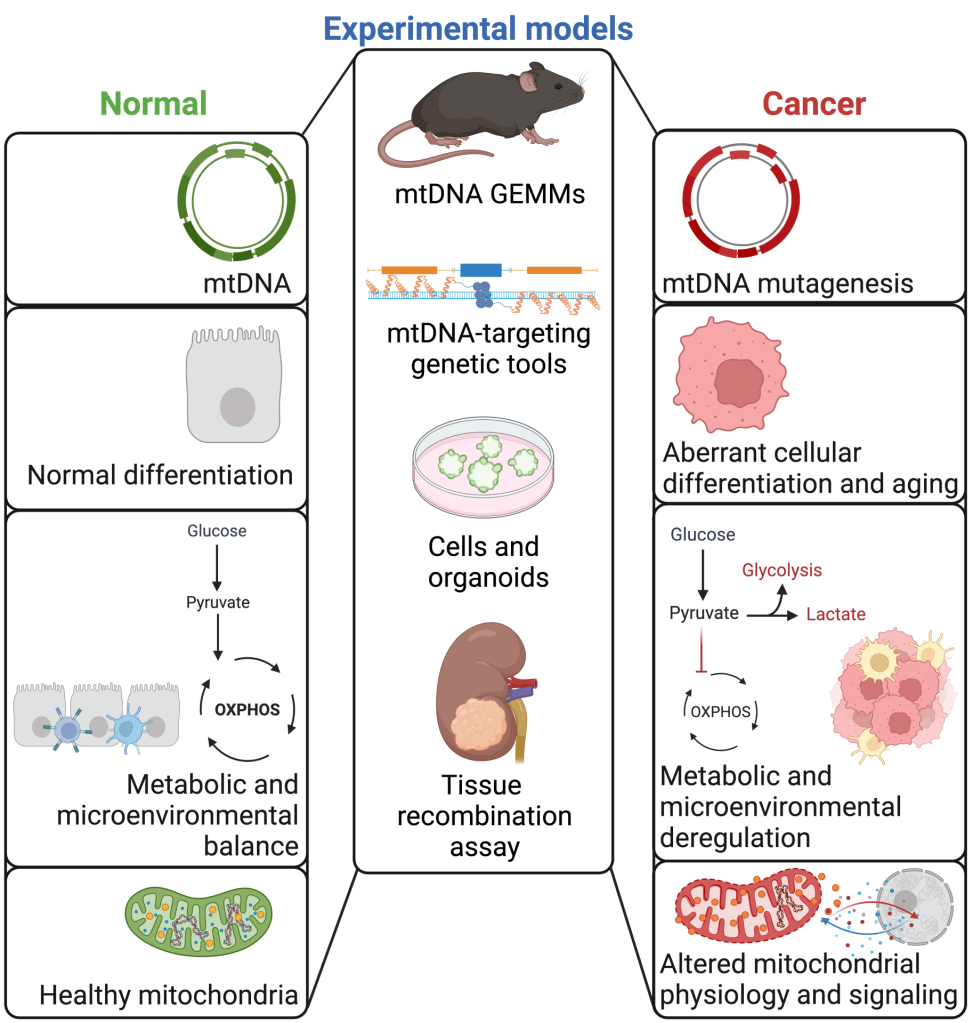
Our laboratory uses a plethora of pre-clinical models, which recapitulate the genetics, progression and heterogeneity of human prostate tumors as well as genome base editing tools to readily characterize the function of pathogenic mtDNA alterations. We are particularly interested in determining the functional impact and clinical relevance of genetic and metabolic factors for prostate cancer progression and disparities. By understanding how these alterations confer specific vulnerabilities to more aggressive tumors, his studies aim at developing novel precision medicine strategies that can improve patient care.
Focus Areas:
- Cancer and Mitochondrial genomics
- Mitochondrial homeostasis and signaling
- Epithelial and Tumor Microenvironment (TME) metabolic remodeling
- Precision medicine therapeutics
